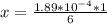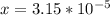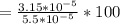Chemistry, 15.07.2020 01:01 cornpops4037
The average human body contains 5.00 L of blood with a Fe2+ concentration of 1.10×10−5 M . If a person ingests 9.00 mL of 21.0 mM NaCN, what percentage of iron(II) in the blood would be sequestered by the cyanide ion?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 18:30, sarahbug56
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 23:30, znewkirk4741
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 01:30, sheldonwaid4278
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
The average human body contains 5.00 L of blood with a Fe2+ concentration of 1.10×10−5 M . If a pers...
Questions in other subjects:


English, 29.10.2020 14:00

Social Studies, 29.10.2020 14:00

History, 29.10.2020 14:00

Mathematics, 29.10.2020 14:00

Mathematics, 29.10.2020 14:00

Biology, 29.10.2020 14:00

Physics, 29.10.2020 14:00

Mathematics, 29.10.2020 14:00

Chemistry, 29.10.2020 14:00

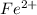
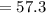 %
%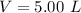
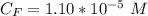
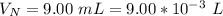
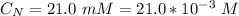
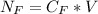
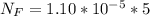
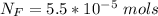
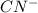 ingested is mathematically evaluated as
ingested is mathematically evaluated as 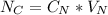
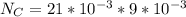

![Fe^{2+} + 6 CN^{-} \to [Fe(CN)_6]^{2-}](/tpl/images/0706/5120/f86b8.png)
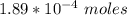 of
of