
Chemistry, 14.07.2020 01:01 eggemotions
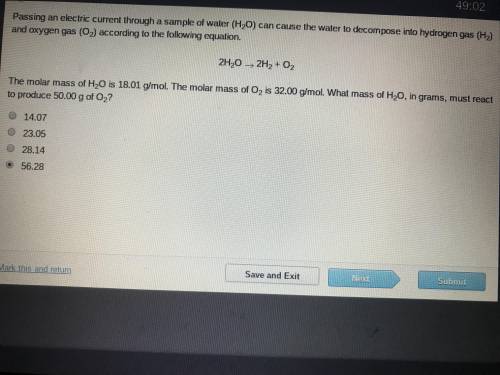
The molar mass of H2O is 18.01 g/mol. The molar mass of O2 is 32.00 g/mol. What mass of H2O, ins grams, must react to produce 50.00 g of O2

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Bradgarner772
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1


Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1
You know the right answer?
The molar mass of H2O is 18.01 g/mol. The molar mass of O2 is 32.00 g/mol. What mass of H2O, ins gra...
Questions in other subjects:



Health, 02.12.2021 21:40

Mathematics, 02.12.2021 21:40




Mathematics, 02.12.2021 21:40

Mathematics, 02.12.2021 21:40

History, 02.12.2021 21:40



