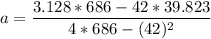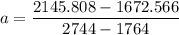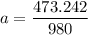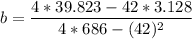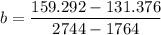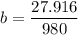Chemistry, 13.07.2020 23:01 Cheesygodxx
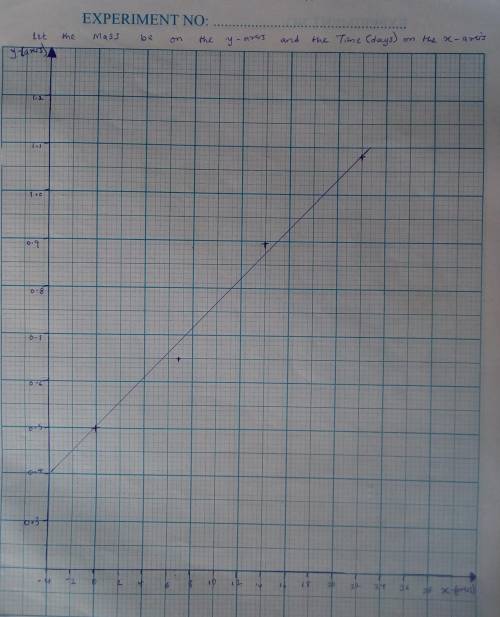
A seed of CuSO4.5H20 with a mass of 0.500 g was carefully placed into a saturated solution of copper (II) sulfate. After 7 days the mass of the seed crystal was determined to be 0.648 g. After 14 days the mass of the crystal increased to 0.899 g and after 21 days the mass of the crystal was found to be 1.081 g. Make a plot of mass vs time (days) and extrapolate to predict what would be the mass of the crystal in 28 days if the growth is linear. Include labels and units on each axis.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, Queenquestion5967
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3


Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
A seed of CuSO4.5H20 with a mass of 0.500 g was carefully placed into a saturated solution of copper...
Questions in other subjects:

Health, 25.05.2021 01:40


Social Studies, 25.05.2021 01:40


Mathematics, 25.05.2021 01:40

Mathematics, 25.05.2021 01:40




Mathematics, 25.05.2021 01:40

 :42 3.128 39.823 686
:42 3.128 39.823 686