
Chemistry, 14.07.2020 21:01 hellothere69
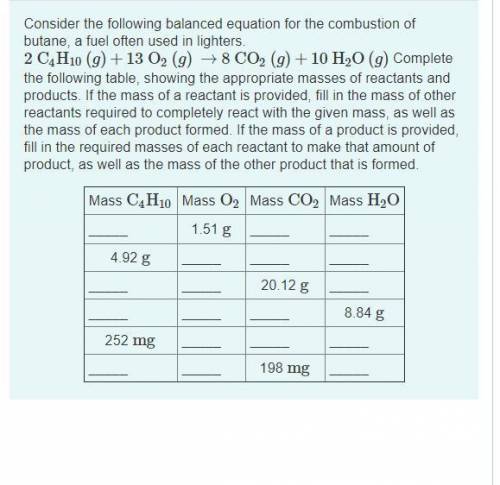
2C4H10(g)+13O2(g)→8CO2(g)+10H2O(g) Complete the following table

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, jamccoy3335
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 00:40, petriajack8375
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3


Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2
You know the right answer?
2C4H10(g)+13O2(g)→8CO2(g)+10H2O(g) Complete the following table
...
...
Questions in other subjects:


Mathematics, 28.12.2020 01:40

English, 28.12.2020 01:40


Mathematics, 28.12.2020 01:40

Social Studies, 28.12.2020 01:40

Chemistry, 28.12.2020 01:40


Mathematics, 28.12.2020 01:40



