
Chemistry, 08.07.2020 14:01 katier9407
It takes to break an carbon-chlorine single bond. Calculate the maximum wavelength of light for which an carbon-chlorine single bond could be broken by absorbing a single photon. Round your answer to significant digits.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, omoaye
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3


Chemistry, 23.06.2019 01:00, birdman2540
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
It takes to break an carbon-chlorine single bond. Calculate the maximum wavelength of light for whic...
Questions in other subjects:





History, 13.10.2020 19:01

Mathematics, 13.10.2020 19:01




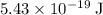 of energy to break one
of energy to break one  single bond.
single bond. 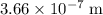 (in vacuum.) That's the same as
(in vacuum.) That's the same as 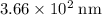 (rounded to three significant figures.)
(rounded to three significant figures.)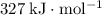 (note that the exact value can varies across sources.) In other words, it would take approximately
(note that the exact value can varies across sources.) In other words, it would take approximately 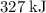 of energy to break one mole of these bonds.
of energy to break one mole of these bonds. 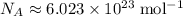 gives the number of
gives the number of  .
.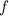 of a photon to its energy
of a photon to its energy 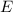 :
: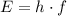 .
.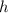 here represents the Planck Constant:
here represents the Planck Constant: 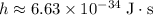 .
.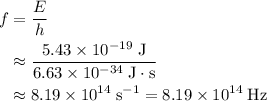 .
.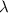 of a photon with a frequency of approximately
of a photon with a frequency of approximately 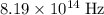 ? The exact answer to that depends on the medium that this photon is travelling through. To be precise, the exact answer depends on the speed of light in that medium:
? The exact answer to that depends on the medium that this photon is travelling through. To be precise, the exact answer depends on the speed of light in that medium: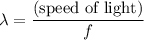 .
.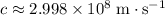 . Therefore, the wavelength of that
. Therefore, the wavelength of that 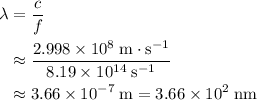 .
.

