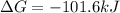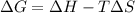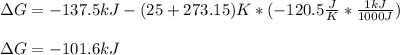Chemistry, 08.07.2020 20:01 michelle7511
Consider the following reaction: C2H4(g)+H2(g)→C2H6(g) ΔH=−137.5kJ; ΔS=−120.5J/K Calculate ΔG at 25 ∘C and determine whether the reaction is spontaneous. Express the free energy change in joules to four significant figures.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:00, edgar504xx
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 01:30, nikonee
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
Consider the following reaction: C2H4(g)+H2(g)→C2H6(g) ΔH=−137.5kJ; ΔS=−120.5J/K Calculate ΔG at 25...
Questions in other subjects:

English, 26.01.2022 14:00



Social Studies, 26.01.2022 14:00

Mathematics, 26.01.2022 14:00


Computers and Technology, 26.01.2022 14:00



Mathematics, 26.01.2022 14:00