Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:00, genyjoannerubiera
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 22.06.2019 23:00, tovarclaudia055
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 00:00, savyblue1724707
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
You know the right answer?
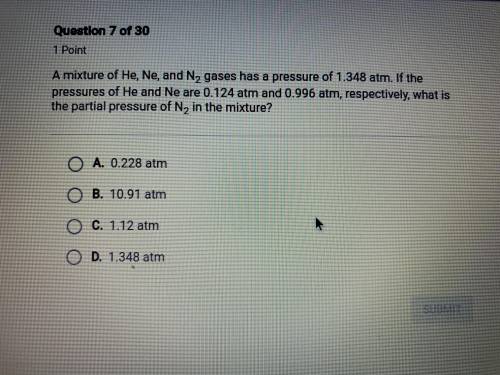
a mixture of He, Ne, and N2 gases has a pressure of 1.348 atm. if the pressures of He and Ne are 0.1...
Questions in other subjects:


Mathematics, 25.08.2019 16:00

History, 25.08.2019 16:00


Mathematics, 25.08.2019 16:00


Computers and Technology, 25.08.2019 16:00

History, 25.08.2019 16:00

Mathematics, 25.08.2019 16:00