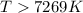Chemistry, 08.07.2020 02:01 ordonez9029
For the reaction
N2(g) + O2(g)→2 NO(g) H° = 181 kJ and S° = 24.9 J/K G°
The equilibrium constant, K, would be greater than 1 at temperatures (above or below).. Kelvin.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, raizagisselle1694
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 16:40, westball101
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
For the reaction
N2(g) + O2(g)→2 NO(g) H° = 181 kJ and S° = 24.9 J/K G°
The equilibriu...
The equilibriu...
Questions in other subjects:



History, 25.03.2020 16:59







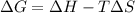
 = enthalpy change = 181 kJ/mol = 181000 J/mol (1kJ=1000J)
= enthalpy change = 181 kJ/mol = 181000 J/mol (1kJ=1000J) = entropy change= 24.9 J/Kmol
= entropy change= 24.9 J/Kmol
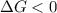
 < 0,
< 0,  >
>