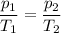Chemistry, 08.07.2020 01:01 jilliandantuma84
Determine the new temperature of a sample of gas if the pressure changes from 0.500 atm to 0.750 atm, and the gas had an initial temperature of 315 K. The volume and number of gas particles is held constant.
210. K
118 K
2.12 x 10-3 K
473 K

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, 21brooklynmartin
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2


Chemistry, 22.06.2019 09:30, janetexcoelho
What does the mass of 0.7891 mol of ferric oxide (fe2o3)
Answers: 1

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3
You know the right answer?
Determine the new temperature of a sample of gas if the pressure changes from 0.500 atm to 0.750 atm...
Questions in other subjects:



Mathematics, 27.10.2020 01:20

Mathematics, 27.10.2020 01:20



Mathematics, 27.10.2020 01:20

Mathematics, 27.10.2020 01:20