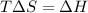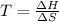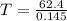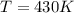Use the reaction l2(s) = 12(9), AH = 62.4 kJ/mol, AS = 0.145 kJ/(mol-K), for
this question
At...


Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, penelopymorales24
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 18:00, ambarpena14
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 23.06.2019 01:00, MrTeriffic
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1
You know the right answer?
Questions in other subjects:




Mathematics, 13.01.2021 20:10

Mathematics, 13.01.2021 20:10

Mathematics, 13.01.2021 20:10

Mathematics, 13.01.2021 20:10

Social Studies, 13.01.2021 20:10

Mathematics, 13.01.2021 20:10

Social Studies, 13.01.2021 20:10

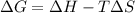
 = Gibbs free energy
= Gibbs free energy  = enthalpy change = 62.4 kJ/mol
= enthalpy change = 62.4 kJ/mol![\Delta S = entropy change = 0.145 kJ/mol KT = temperature in Kelvin[tex]\Delta G](/tpl/images/0702/3677/b9e38.png) = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous