
Carbon monoxide (CO) reacts with hydrogen (H2) to form methane (CH4) and water (H20).
CO(g) + 3H2(g) + CH4(g)+H20(9)
The reaction is at equilibrium at 1,000 K. The equilibrium constant of the reaction is 3.90. At equilibrium, the concentrations are as
follows.
[CO] = 0.30 M
[H2] = 0.10 M
[H20] = 0.020 M
What is the equilibrium concentration of CH, expressed in scientific notation?
0.0059
5.9 x 10-2
0.059
5.9 x 102

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, kayleg907436
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1


Chemistry, 22.06.2019 07:30, superfly903
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 11:40, Wemaybewrong
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1
You know the right answer?
Carbon monoxide (CO) reacts with hydrogen (H2) to form methane (CH4) and water (H20).
CO(g) + 3H2(g...
Questions in other subjects:

English, 19.01.2021 17:40







Mathematics, 19.01.2021 17:40


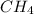 , expressed in scientific notation is
, expressed in scientific notation is 
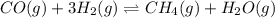
![K_c=\frac{[CH_4]\times [H_2O]}{[CO]\times [H_2]^3}](/tpl/images/0702/2496/1cdb8.png)




