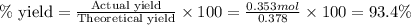Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 23.06.2019 00:30, emilylizbeth12334
Which of the following best describes technology a. something created for only scientists to use b. the method of thinking that scientists use. c. the application of engineering to create useful products. c. a scientific idea
Answers: 1
You know the right answer?
A reaction of 41.9 g of Na and 30.3 g of Br2 yields 36.4 g of NaBr . What is the percent yield?
2Na...
Questions in other subjects:


English, 25.04.2020 04:51



Mathematics, 25.04.2020 04:51




Social Studies, 25.04.2020 04:51

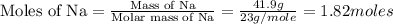
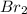
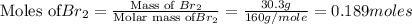
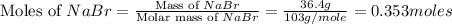
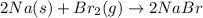
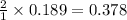 moles of Sodium
moles of Sodium