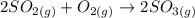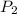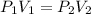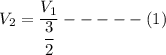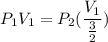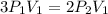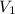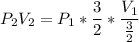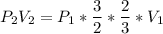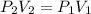Chemistry, 05.07.2020 14:01 jakobrobinette
Consider this reaction at equilibrium at a total pressure P1: 2SO2(g) + O2(g) → 2SO3(g) Suppose the volume of this system is compressed to one-half its initial volume and then equilibrium is reestablished. The new equilibrium total pressure will be:

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, micahwilkerson9495
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2


Chemistry, 22.06.2019 22:30, darkshaders11
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
Consider this reaction at equilibrium at a total pressure P1: 2SO2(g) + O2(g) → 2SO3(g) Suppose th...
Questions in other subjects:



Mathematics, 08.07.2019 23:00