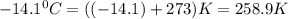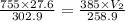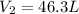Chemistry, 04.07.2020 14:01 aeshaalhemri
A weather balloon is inflated to a volume of 27.6 L at a pressure of 755 mmHg and a temperature of 29.9 ∘C. The balloon rises in the atmosphere to an altitude where the pressure is 385 mmHg and the temperature is -14.1 ∘C. Assuming the balloon can freely expand, calculate the volume of the balloon at this altitude.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, sleimanabir
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 23.06.2019 06:30, OsoDeOro7968
Which of these natural resources is non-renewable a. corn b. wind c. geothermal d. natural gas
Answers: 2

Chemistry, 23.06.2019 09:00, hunterwilliams375
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2
You know the right answer?
A weather balloon is inflated to a volume of 27.6 L at a pressure of 755 mmHg and a temperature of 2...
Questions in other subjects:

Mathematics, 12.11.2020 17:10




Mathematics, 12.11.2020 17:10





Geography, 12.11.2020 17:10

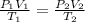
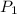 = initial pressure of gas = 755 mm Hg
= initial pressure of gas = 755 mm Hg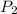 = final pressure of gas (at STP) = 385 mm Hg
= final pressure of gas (at STP) = 385 mm Hg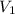 = initial volume of gas = 27.6 L
= initial volume of gas = 27.6 L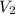 = final volume of gas = ?
= final volume of gas = ?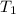 = initial temperature of gas =
= initial temperature of gas = 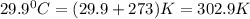
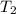 = final temperature of gas =
= final temperature of gas =