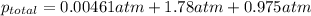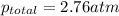Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 23.06.2019 01:00, liamgreene90
The time that is taken by neptune once around the sun is called
Answers: 1
You know the right answer?
The partial pressures of the gases in a mixture are 0.00461 atm O2, 1.78 atm
N2, and 0.975 atm Ar....
Questions in other subjects:

Computers and Technology, 29.04.2021 09:40

English, 29.04.2021 09:40

German, 29.04.2021 09:40


Mathematics, 29.04.2021 09:40




Computers and Technology, 29.04.2021 09:40

Mathematics, 29.04.2021 09:40

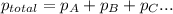
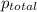 =total pressure of gases = ?
=total pressure of gases = ?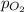 = partial pressure of oxygen = 0.00461 atm
= partial pressure of oxygen = 0.00461 atm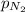 = partial pressure of nitrogen = 1.78 atm
= partial pressure of nitrogen = 1.78 atm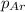 = partial pressure of argon = 0.975 atm
= partial pressure of argon = 0.975 atm