A proposed mechanism for the reaction of NO2 and CO is
Step 1: Slow, endothermic:
2 NO2 (g) → NO (g) + NO3 (g)
Step 2: Fast, exothermic:
NO3 (g) + CO (g) → NO2 (g) + CO2 (g)
Overall reaction, exothermic:
NO2 (g) + CO (g) → NO (g) + CO2 (g)
a. Identify each of the following as a reactant, product, or intermediate:
1. NO2(g)
2. CO(g)
3. NO3(g)
4. CO2(g)
5. NO(g)
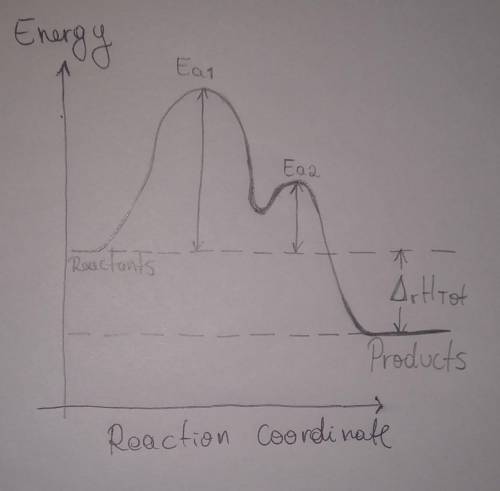
b. Draw a reaction coordinate diagram for this reaction. Indicate on this drawing the activation energy for each step and the overall enthalpy change.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, daniellekennedy05
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 07:30, gwenparks
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

You know the right answer?
A proposed mechanism for the reaction of NO2 and CO is
Step 1: Slow, endothermic:
2 NO2 (g)...
2 NO2 (g)...
Questions in other subjects:

Mathematics, 11.05.2021 22:20



Spanish, 11.05.2021 22:20


English, 11.05.2021 22:20



Mathematics, 11.05.2021 22:20