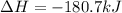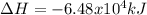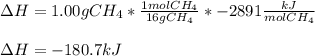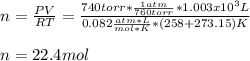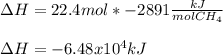Chemistry, 04.07.2020 02:01 khikhi1705
Consider the following reaction:
CH4 +2O2 → CO2 + 2H2O. ΔH= -2891 kJ
Calculate the enthalpy change for each of the following cases:
a. 1.00 g methane is burned in excess oxygen.
b. 1.00 3 10^3 L methane gas at 740. torr and 258°C are burned in excess oxygen.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 09:30, jewelz5887
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1
You know the right answer?
Consider the following reaction:
CH4 +2O2 → CO2 + 2H2O. ΔH= -2891 kJ
Calculate the enth...
Calculate the enth...
Questions in other subjects:



Social Studies, 27.06.2019 10:30



Geography, 27.06.2019 10:30

English, 27.06.2019 10:30


Physics, 27.06.2019 10:30