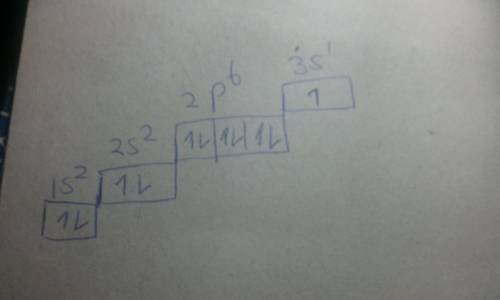
Chemistry, 04.07.2020 01:01 GreenHerbz206
The excited state of an atom is one in which an electron is in a higher energy level (or sublevel) than it is in its ground state (the "normal" electron configuration). The energy level to which the electron transitions must be an allowable energy level (for example an electron cannot transition from a ls to a lp sublevel because the lp sublevel does not exist). An excited state for Na, above its ground state, is [Ne]3p'. When the electron transitions back to the ground state, it emits the yellow-orange light characteristic of Na. The energy of this photon of light is equal to the energy difference between the 3p and the orbital in which the electron is located in the ground state.
a) Draw the ground state electron configuration and orbital filling diagram for Na in its ground state.
b) Another excited state for Na is [Ne]4p'. Which of the following could describe the radiation emitted by the Na atom as it transitions back to the ground state: UV light, the same yellow orange light as described above, red light, or infrared light? Explain.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, keiracoles
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1


Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
The excited state of an atom is one in which an electron is in a higher energy level (or sublevel) t...
Questions in other subjects:





Social Studies, 10.10.2019 14:00



English, 10.10.2019 14:00