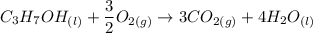The combustion of 1.685 g of propanol (C3H7OH) increases the temperature of a bomb calorimeter from 298.00 K to 302.16 K. The heat capacity of the bomb calorimeter is 13.60 kJ/K . Determine ΔH for the combustion of propanol to carbon dioxide gas and liquid water. g

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 17:30, shookiegriffin
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 23.06.2019 06:30, jamarstand
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1

Chemistry, 23.06.2019 07:00, jaydenboi604
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1
You know the right answer?
The combustion of 1.685 g of propanol (C3H7OH) increases the temperature of a bomb calorimeter from...
Questions in other subjects:

English, 29.06.2019 20:00


Mathematics, 29.06.2019 20:00

Social Studies, 29.06.2019 20:00

History, 29.06.2019 20:00



Mathematics, 29.06.2019 20:00

Mathematics, 29.06.2019 20:00

Biology, 29.06.2019 20:00