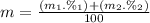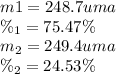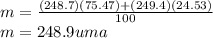Chemistry, 02.10.2019 14:50 Jsquad8879
Calculate the average atomic mas of an unknown element that has two naturally-occurring isotopes.
the first isotope occurs 75.47% of the time and has a mass of 248.7 a. m.u.
the second isotope occurs 24.53% of the time and has a mass of 249.4 a. m.u.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, rosie20052019
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 23:30, lizdeleon248
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 03:00, winterblanco
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3
You know the right answer?
Calculate the average atomic mas of an unknown element that has two naturally-occurring isotopes.
Questions in other subjects:

Physics, 22.07.2019 13:20




History, 22.07.2019 13:20



Mathematics, 22.07.2019 13:20

Biology, 22.07.2019 13:20