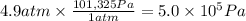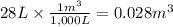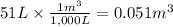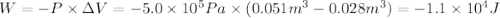Chemistry, 01.07.2020 23:01 thecoolgirl02
Calculate the work (kJ) done during a reaction in which the internal volume expands from 28 L to 51 L against an outside pressure of 4.9 atm. Calculate the work (kJ) done during a reaction in which the internal volume expands from 28 L to 51 L against an outside pressure of 4.9 atm. 11 kJ -11 kJ -39 kJ 39 kJ 0 kJ; No work is done.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, chinadoll24
Which of the following is an example of a parasite?
Answers: 3

Chemistry, 23.06.2019 02:30, hailee232
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1

Chemistry, 23.06.2019 03:30, alvfran1041
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1

Chemistry, 23.06.2019 13:00, madelyngv97
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Calculate the work (kJ) done during a reaction in which the internal volume expands from 28 L to 51...
Questions in other subjects:



Mathematics, 08.02.2021 01:40

Mathematics, 08.02.2021 01:40


Health, 08.02.2021 01:40

History, 08.02.2021 01:40


English, 08.02.2021 01:40