
Chemistry, 01.07.2020 18:01 lauren9261
Consider the reaction: 2 NO(g) + 5 H2(g) → 2 NH3(g) + 2 H2O(g)A reaction mixture initially contains 5 moles of NO and 10 moles of H2. Which set of amounts best represents the mixture after the reactants have reacted as completely as possible?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, hannah5143
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 21.06.2019 22:40, babygirlqueen5588
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 00:00, nyasiasaunders1234
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2
You know the right answer?
Consider the reaction: 2 NO(g) + 5 H2(g) → 2 NH3(g) + 2 H2O(g)A reaction mixture initially contains...
Questions in other subjects:

Mathematics, 26.03.2021 18:20

Mathematics, 26.03.2021 18:20

Chemistry, 26.03.2021 18:20



Mathematics, 26.03.2021 18:20

Mathematics, 26.03.2021 18:20


Chemistry, 26.03.2021 18:20

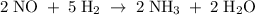
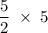 moles Hydrogen
moles Hydrogen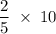 moles NO
moles NO


