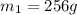Chemistry, 30.06.2020 18:01 6710000831
A silver block, initially at 56.1 ∘C, is submerged into 100.0 g of water at 24.0 ∘C, in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 28.0∘C. What is the mass of the silver block?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, kathleensumter4913
219 grams of iron (iii) oxide reacts with excess carbon according to the reaction equation shown below. fe2o3 + c → fe + co2 after a scientist performs the chemical reaction they find the actual yield of iron to be 57.4 grams. calculate the percent yield of this chemical reaction.
Answers: 1

Chemistry, 22.06.2019 04:10, tishfaco5000
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

You know the right answer?
A silver block, initially at 56.1 ∘C, is submerged into 100.0 g of water at 24.0 ∘C, in an insulated...
Questions in other subjects:

Mathematics, 20.04.2020 20:29

Mathematics, 20.04.2020 20:29

Mathematics, 20.04.2020 20:29

Mathematics, 20.04.2020 20:29



Mathematics, 20.04.2020 20:29



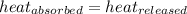
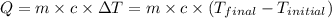
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0697/4196/09236.png) .................(1)
.................(1)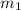 = mass of silver = ?
= mass of silver = ?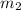 = mass of water = 100.0 g
= mass of water = 100.0 g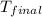 = final temperature =
= final temperature = 
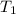 = temperature of silver =
= temperature of silver = 
 = temperature of water =
= temperature of water = 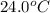
 = specific heat of silver =
= specific heat of silver = 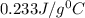
 = specific heat of water=
= specific heat of water= 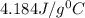
![-m_1\times 0.233\times (28.0-56.1)=[100.0\times 4.184\times (28.0-24.0)]](/tpl/images/0697/4196/c0833.png)