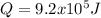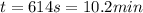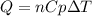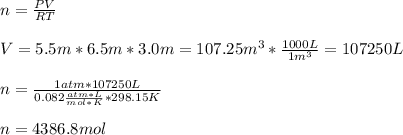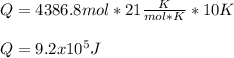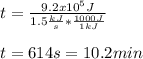The heat capacity of air is much smaller than that of water, and relatively modest amounts of heat are needed to change its temperature. This is one of the reasons why desert region, although very hot during the day, are bitterly cold at night. The heat capacity of air at room temperature and pressure is appoximately 21 J/K*mol. How much energy is required to raise the temperature of a room of dimensions 5.5m x 6.5m x 3.0m by 10 degrees Celsius? If losses are neglected, how long will it take a heater rated at 1.5 kW to achieve that increase given that 1 W = 1 J/s?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:40, Snowball080717
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 23:00, emilyphillips1681
If two identical atoms are bonded, what kind of molecule is formed
Answers: 1
You know the right answer?
The heat capacity of air is much smaller than that of water, and relatively modest amounts of heat a...
Questions in other subjects:

Physics, 06.06.2020 15:58


Physics, 06.06.2020 15:58

Chemistry, 06.06.2020 15:58





Mathematics, 06.06.2020 15:58

History, 06.06.2020 15:58