
Chemistry, 29.06.2020 04:01 hanchinsko12
How many moles of each product form when the given amount of each reactant completely reacts. C3H8(g)+5O2yields 3CO2(g)+4H2O(g). 4.6 moles of C3H8

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:40, jaueuxsn
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 12:30, pup88
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2
You know the right answer?
How many moles of each product form when the given amount of each reactant completely reacts. C3H8(g...
Questions in other subjects:


Mathematics, 20.09.2020 02:01



Chemistry, 20.09.2020 02:01

Advanced Placement (AP), 20.09.2020 02:01



History, 20.09.2020 02:01

English, 20.09.2020 02:01

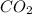 and 18.4 moles of
and 18.4 moles of 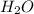 will be produced
will be produced
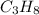
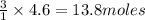 of
of 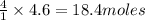 of
of 

