Answers: 3


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 03:00, KindaSmartPersonn
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2

Chemistry, 23.06.2019 08:00, george27212
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1
You know the right answer?
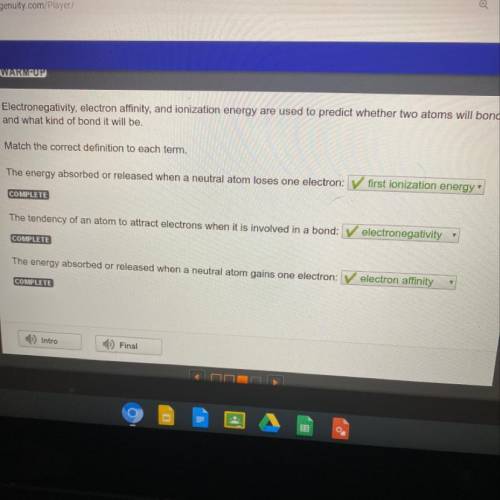
Electronegativity, electron
affinity, and ionization energy are used to predict whether two atoms w...
Questions in other subjects:




Mathematics, 13.11.2021 06:10


Mathematics, 13.11.2021 06:10


Mathematics, 13.11.2021 06:10