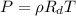Chemistry, 27.06.2020 19:01 NNopeNNopeNNope
On a hot summer day, the density of air at atmospheric pressure at 35.5°C is 1.1970 kg/m3. (a) What is the number of moles contained in 1.00 m3 of an ideal gas at this temperature and

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, mamasmontoya
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2


Chemistry, 22.06.2019 04:30, only1cache
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3
You know the right answer?
On a hot summer day, the density of air at atmospheric pressure at 35.5°C is 1.1970 kg/m3. (a) What...
Questions in other subjects:

Mathematics, 05.10.2021 23:10




Social Studies, 05.10.2021 23:10



Mathematics, 05.10.2021 23:10

Social Studies, 05.10.2021 23:10