
The same reaction, with exactly the same amount of reactant, is conducted in a bomb calorimeter and in a coffee-cup calorimeter. In one measurement, qrxn=−13.3qrxn=−13.3 kJkJ in the other qrxn=−11.9qrxn=−11.9 kJkJ. Which value was obtained in the bomb calorimeter? (Assume that the reaction has a positive ΔVΔV in the coffee-cup calorimeter.)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, umimgoingtofail
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 21.06.2019 21:00, yousifgorgees101
The earth's moon is unusually large. two popular theories of the moon's origin include the "sister world" hypothesis, which states that the moon formed from the same materials as the earth, near enough to the earth that they fell into orbit around each other. a second theory is the "capture" hypothesis, in which the moon formed elsewhere in the solar system, and the earth's gravity pulled it into its orbit. studies of what the moon is made of indicate that some of its materials had to come from the earth or from the same area of the solar system where the earth had formed. at the same time, the moon does not contain much of the material that makes up the earth's core, so the moon could not have formed from the same materials as the earth. how do the two facts above affect the described theories of the moon's origin? a. they show that scientists will never agree on where the moon came from. b. they show that more experiments on moon formation need to be done. c. they show that no theory accounts for the existence of the moon. d. they show that neither theory is complete and entirely correct.
Answers: 1

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 22:30, teagan56
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
The same reaction, with exactly the same amount of reactant, is conducted in a bomb calorimeter and...
Questions in other subjects:


Mathematics, 14.08.2020 01:01


Chemistry, 14.08.2020 01:01






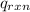 = -13.3 KJ belongs to the bomb calorimeter
= -13.3 KJ belongs to the bomb calorimeter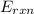 of the reaction, the work of the reaction
of the reaction, the work of the reaction 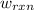 and the heat of the reaction
and the heat of the reaction 

