
Chemistry, 21.10.2019 18:00 selamh1999
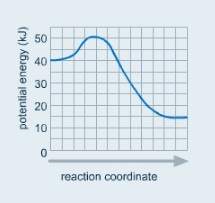
Look at the potential energy diagram for a chemical reaction.
which statement correctly describes the energy changes that occur in the forward reaction?
the activation energy is 10 kj and the reaction is exothermic.
the activation energy is 10 kj and the reaction is endothermic.
the activation energy is 50 kj and the reaction is exothermic.
the activation energy is 50 kj and the reaction is endothermic.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3

Chemistry, 22.06.2019 11:30, elijah1090
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2


Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
Look at the potential energy diagram for a chemical reaction.
which statement correctly...
which statement correctly...
Questions in other subjects:

History, 07.03.2021 14:00

Mathematics, 07.03.2021 14:00


Chemistry, 07.03.2021 14:00

Physics, 07.03.2021 14:00



English, 07.03.2021 14:00


World Languages, 07.03.2021 14:00



