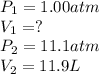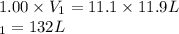Chemistry, 27.06.2020 07:01 reeseebaby89
Calculate the volume of a balloon that could be filled at 1.00 atm with the helium in a 11.9 L gas cylinder in which the pressure is 11.1 atm (assume no temperature change).

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, carsonjohnsonn
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3
You know the right answer?
Calculate the volume of a balloon that could be filled at 1.00 atm with the helium in a 11.9 L gas c...
Questions in other subjects:


Mathematics, 27.01.2020 20:31


History, 27.01.2020 20:31


Social Studies, 27.01.2020 20:31

Mathematics, 27.01.2020 20:31

Social Studies, 27.01.2020 20:31

History, 27.01.2020 20:31

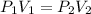
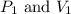 are initial pressure and volume.
are initial pressure and volume.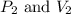 are final pressure and volume.
are final pressure and volume.