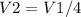Chemistry, 27.06.2020 06:01 whyidkmyself
Be sure to answer all parts. What is the effect of each of the following on the volume of 1 mol of an ideal gas? (a) The pressure changes from 760 torr to 202 kPa, and the temperature changes from 37°C to 155 K. The volume increases by a factor of 2. The volume increases by a factor of 4. The volume decreases by a factor of 2. The volume decreases by a factor of 4. The volume remains the same. (b) The temperature changes from 305 K to 32°C, and the pressure changes from 2 atm to 101 kPa. The volume increases by a factor of 2. The volume increases by a factor of 4. The volume decreases by a factor of 2. The volume decreases by a factor of 4. The volume remains the same.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1


Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
Be sure to answer all parts. What is the effect of each of the following on the volume of 1 mol of a...
Questions in other subjects:



Social Studies, 05.05.2020 14:54

Mathematics, 05.05.2020 14:54


Mathematics, 05.05.2020 14:54

History, 05.05.2020 14:54