Chemistry, 26.06.2020 17:01 villafana36
A 650.0 mL solution contains 125 grams of glucose (C6H12O6). If the molar mass of C6H12O6 is 180.16 g/mol, what is the molarity of this solution? answer options are 0.0106 M C6H12O6 0.0195 M C6H12O6 1.07 M C6H12O6 1.92 M C6H12O6
need help ASAP
will mark brainlest

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, aylengarcia090
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
A 650.0 mL solution contains 125 grams of glucose (C6H12O6). If the molar mass of C6H12O6 is 180.16...
Questions in other subjects:


Chemistry, 02.12.2020 20:40

Chemistry, 02.12.2020 20:40

Mathematics, 02.12.2020 20:40

Mathematics, 02.12.2020 20:40

Chemistry, 02.12.2020 20:40


Mathematics, 02.12.2020 20:40

Mathematics, 02.12.2020 20:40

Spanish, 02.12.2020 20:40

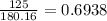 mol de glucosa
mol de glucosa