
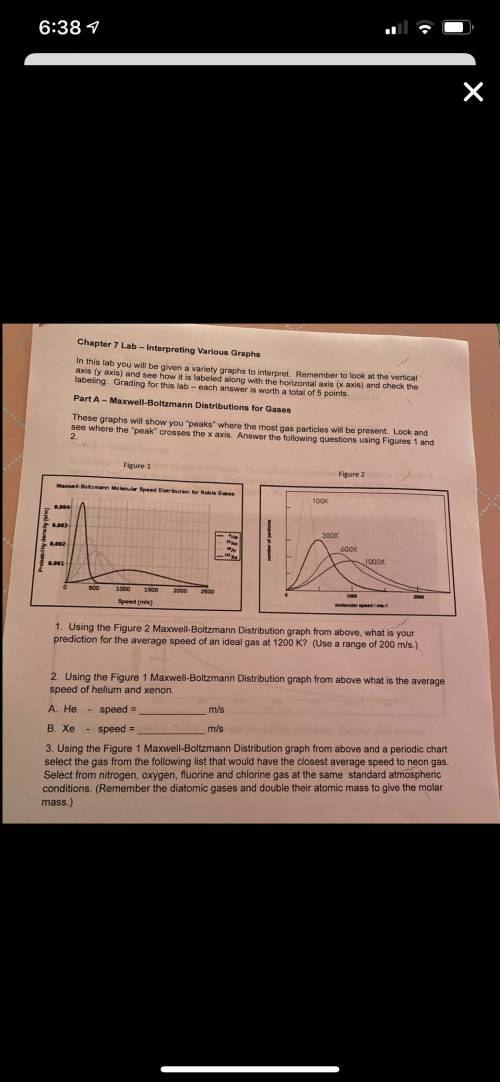
Using the Figure 1 Maxwell-Boltzmann Distribution graph from above and a periodic chart select the gas from the following list that would have the closest average speed to neon gas. Select from nitrogen, oxygen, fluorine and chlorine gas at the same standard atmospheric conditions. (Remember the diatomic gases and double their atomic mass to give the molar mass.) Using the Figure 1 Maxwell-Boltzmann Distribution graph from above and a periodic chart select the gas from the following list that would have the slowest average speed with all at the same standard atmospheric conditions. Select from carbon dioxide, argon, nitrogen and chlorine.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 05:50, Makoshark6887
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
Using the Figure 1 Maxwell-Boltzmann Distribution graph from above and a periodic chart select the g...
Questions in other subjects:


Business, 10.12.2021 19:40

English, 10.12.2021 19:40


Mathematics, 10.12.2021 19:40

History, 10.12.2021 19:40

Biology, 10.12.2021 19:40

Geography, 10.12.2021 19:40




