Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:20, datboyjulio21
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2


You know the right answer?
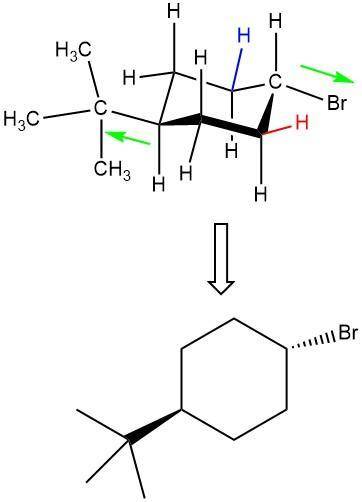
Which isomer of 1-bromo-4-tert-butylcyclohexane reacts faster when refluxed with potassium tert-buto...
Questions in other subjects:

History, 25.09.2019 20:20

History, 25.09.2019 20:20


Health, 25.09.2019 20:20

Biology, 25.09.2019 20:20


English, 25.09.2019 20:20

Mathematics, 25.09.2019 20:20