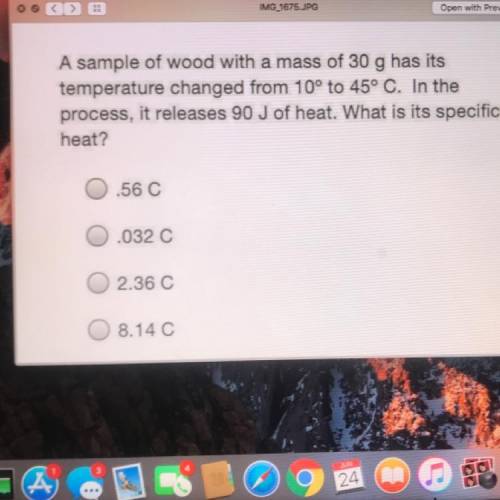
A sample of wood with a mass of 30 g has its
temperature changed from 10° to 45° C. In the
pr...

Chemistry, 26.06.2020 15:01 vinp190p9zekn
A sample of wood with a mass of 30 g has its
temperature changed from 10° to 45° C. In the
process, it releases 90 J of heat. What is its specific
heat?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mayamabjishovrvq9
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 10:00, Cythina2007
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

You know the right answer?
Questions in other subjects:


Physics, 21.05.2020 01:57






Mathematics, 21.05.2020 01:57

Mathematics, 21.05.2020 01:57

History, 21.05.2020 01:57



