Answers: 3


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 01:30, rubyr9975
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1

Chemistry, 23.06.2019 01:30, koggebless
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
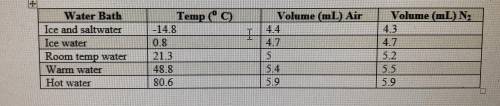
If the atmospheric pressure in the laboratory is 1.2 atm, how many moles of gas were in each syringe...
Questions in other subjects:



Mathematics, 28.03.2020 00:00


Mathematics, 28.03.2020 00:00

Mathematics, 28.03.2020 00:00

Mathematics, 28.03.2020 00:00

History, 28.03.2020 00:00