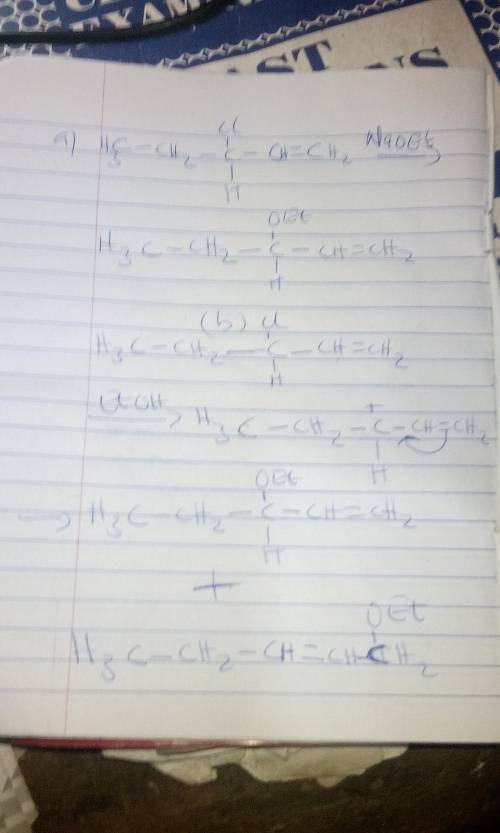Chemistry, 26.06.2020 16:01 tamyrareaves12
3-Chloro-1-pentene reacts with sodium ethoxide in ethanol to produce 3-ethoxy-1-pentene. The reaction is second order, first order in 3-chloro-1-pentene and first order in sodium ethoxide. In the absence of sodium ethoxide, 3-chloro-1-pentene reacts with ethanol to produce both 3-ethoxy-1-pentene and 1-ethoxy-2-pentene. The first reaction proceeds via an mechanism. The stereochemistry of the product is . The second reaction proceeds via an mechanism. The stereochemistry of 3-ethoxy-1-pentene is . The stereochemistry of 1-ethoxy-2-pentene is . For the second reaction, draw the structure of the intermediate's resonance contributor that leads to the formation of 3-ethoxy-1-pentene.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
3-Chloro-1-pentene reacts with sodium ethoxide in ethanol to produce 3-ethoxy-1-pentene. The reactio...
Questions in other subjects:


Physics, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01

Social Studies, 05.10.2020 14:01




Mathematics, 05.10.2020 14:01

Chemistry, 05.10.2020 14:01