

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 14:30, Playboycxm
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1


Chemistry, 23.06.2019 00:30, zaniathomasel
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
g a solution is made by mixing 500.0 mL of 0.037980.03798 M Na2sO4 Na2sO4 with 500.0 mL of 0.034280....
Questions in other subjects:










![\rm [Na^{+}]= \text{0.055 12 mol/L}](/tpl/images/0693/9405/43c80.png)
![\rm [HAsO_{4}^{2-}] + [AsO_{4}^{3-}] + [H_{2}AsO_{4}^{-}] + [H_{3}AsO_{4}] = \text{0.018 99 mol/L}](/tpl/images/0693/9405/595ec.png)
![\rm c_{Na^{+}} = 2[Na^{+}]_{Na_{2}HAsO_{4}} + [Na^{+}]_{NaOH}](/tpl/images/0693/9405/0b777.png)
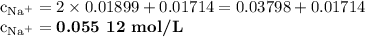
![\rm c_{\text{arsenate}} = [HAsO_{4}^{2-}] + [AsO_{4}^{3-}] + [H_{2}AsO_{4}^{-}] + [H_{3}AsO_{4}]](/tpl/images/0693/9405/5cab1.png)
![\rm [HAsO_{4}^{2-}] + [AsO_{4}^{3-}] + [H_{2}AsO_{4}^{-}] + [H_{3}AsO_{4}] = \textbf{0.018 99 mol/L}](/tpl/images/0693/9405/8974b.png)


