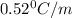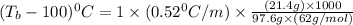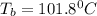Chemistry, 25.06.2020 03:01 michellegregg10
An ethylene glycol solution contains 21.4 g of ethylene glycol (C2H6O2) in 97.6 mL of water. (Assume a density of 1.00 g/mL for water.) Determine the freezing point and boiling point of the solution. (Assume a density of 1.00 g/ mL for water.)

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 23:30, sweaversw9602
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
An ethylene glycol solution contains 21.4 g of ethylene glycol (C2H6O2) in 97.6 mL of water. (Assume...
Questions in other subjects:



Social Studies, 18.07.2019 04:00




Mathematics, 18.07.2019 04:00

Chemistry, 18.07.2019 04:00

Biology, 18.07.2019 04:00

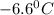 and
and 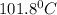 respectively.
respectively.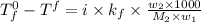
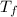 = freezing point of solution = ?
= freezing point of solution = ?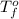 = freezing point of water =
= freezing point of water = 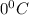
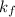 = freezing point constant of water =
= freezing point constant of water = 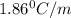
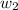 = mass of solute (ethylene glycol) = 21.4 g
= mass of solute (ethylene glycol) = 21.4 g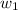 = mass of solvent (water) =
= mass of solvent (water) = 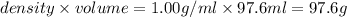
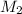 = molar mass of solute (ethylene glycol) = 62g/mol
= molar mass of solute (ethylene glycol) = 62g/mol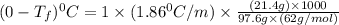
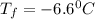
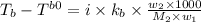
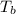 = boiling point of solution = ?
= boiling point of solution = ?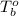 = boiling point of water =
= boiling point of water = 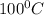
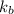 = boiling point constant of water =
= boiling point constant of water =