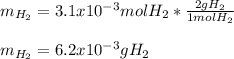Chemistry, 24.06.2020 22:01 keirarae2005
Sometimes in lab we collect the gas formed by a chemical reaction over water. This makes it easy to isolate and measure the amount of gas produced. Suppose H2 the gas evolved by a certain chemical reaction taking place at 40°C is collected over water, using an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 80ml .
Required:
Calculate the mass of H2 that is in the collection tube. Round your answer to significant digits. You can make any normal and reasonable assumption about the reaction conditions and the nature of the gases.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 22:30, vhife4901
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
You know the right answer?
Sometimes in lab we collect the gas formed by a chemical reaction over water. This makes it easy to...
Questions in other subjects:



Mathematics, 09.09.2021 22:50

History, 09.09.2021 22:50