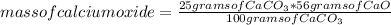Chemistry, 24.06.2020 04:01 torresq6647
How much calcium oxide would be made by the thermal decomposition of 25 grams of calcium carbonate?
CaCO3 -> CaO + CO2

A. 28 grams
B. 12 grams
C. 14 grams
D. 25 grams

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, hammackkatelyn60
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
How much calcium oxide would be made by the thermal decomposition of 25 grams of calcium carbonate?...
Questions in other subjects:

Mathematics, 30.09.2020 06:01




Mathematics, 30.09.2020 06:01

Mathematics, 30.09.2020 06:01

Mathematics, 30.09.2020 06:01



Mathematics, 30.09.2020 06:01