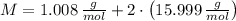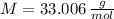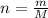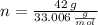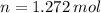Chemistry, 23.06.2020 16:01 leahstubbs
How many moles of \ce{H2O}HX 2 O will be produced from 42.0 \text{ g}42.0 g42, point, 0, start text, space, g, end text of \ce{H2O2}HX 2 OX 2 ?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, nyceastcoast
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 16:30, sbush1412
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u. s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2


Chemistry, 23.06.2019 00:00, alisonsolis155
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
You know the right answer?
How many moles of \ce{H2O}HX 2 O will be produced from 42.0 \text{ g}42.0 g42, point, 0, start text...
Questions in other subjects:

World Languages, 17.10.2021 04:10


Biology, 17.10.2021 04:10


Mathematics, 17.10.2021 04:10

English, 17.10.2021 04:10


English, 17.10.2021 04:10

History, 17.10.2021 04:10

Chemistry, 17.10.2021 04:10

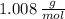 and
and 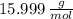 , respectively. A mole is the ratio of current mass of water to molecular weight of water, the latter one is now calculated before computing the amount of moles of water:
, respectively. A mole is the ratio of current mass of water to molecular weight of water, the latter one is now calculated before computing the amount of moles of water: