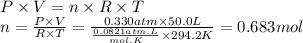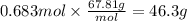Boron trifluoride gas is collected at in an evacuated flask with a measured volume of . When all the gas has been collected, the pressure in the flask is measured to be . Calculate the mass and number of moles of boron trifluoride gas that were collected. Round your answer to significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, NorbxrtThaG
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1


Chemistry, 22.06.2019 09:30, janetexcoelho
What does the mass of 0.7891 mol of ferric oxide (fe2o3)
Answers: 1
You know the right answer?
Boron trifluoride gas is collected at in an evacuated flask with a measured volume of . When all the...
Questions in other subjects:

Mathematics, 22.02.2021 23:10



Engineering, 22.02.2021 23:10



English, 22.02.2021 23:10


Biology, 22.02.2021 23:10