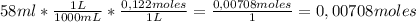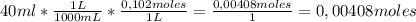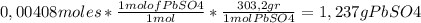Chemistry, 21.10.2019 19:00 connerwoodle8941
A58.0ml sample of a 0.122m potassium sulfate solution is mixed with 40.0ml of a 0.102m lead(ii) acetate solution and this precipitation reaction occurs:
k2so4(aq)+pb(c2h3o2)2(aq)→2kc2h3o2( aq)+pbso4(s)
the solid, pbso4, is collected, dried, and found to have a mass of 0.993g.
determine the:
1) limiting reactant
2) theoretical yield
3) percent yield

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:30, rose888829
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2

Chemistry, 23.06.2019 03:00, cabreradesirae4807
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2

Chemistry, 23.06.2019 04:00, onegirl435
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1
You know the right answer?
A58.0ml sample of a 0.122m potassium sulfate solution is mixed with 40.0ml of a 0.102m lead(ii) acet...
Questions in other subjects:






Mathematics, 05.03.2021 14:00

Mathematics, 05.03.2021 14:00


Chemistry, 05.03.2021 14:00

Mathematics, 05.03.2021 14:00