
Chemistry, 23.06.2020 10:57 mvtthewisdead
The reaction 2 NO(g) ⇌ N2(g) + O2(g) has a value of Keq = 2400 at a temperature of 2000 K. If 0.570 mol of NO(g) is initially placed in a 3.0 L container, calculate the equilibrium concentrations of each gas

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, BaileyElizabethRay
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 05:30, palcochran1313
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 05:30, alexusnicole817
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 05:50, carog24
Astudent made a graph plotting the progress of a reaction over time. the student forgot to label the y-axis of the graph. a graph is shown with two graph lines. one graph line starts at a higher position on the y axis and slopes downwards towards the right. the other graph line starts at a lower position on the y axis and slopes upwards towards the right. the two graph lines stop short of intersecting each other and continue as separate lines which gradually become straight and parallel to the x axis. a vertical line is shown at a point where the two graph lines finally became parallel to the x axis. this vertical line is labeled equilibrium. the title on the x axis is time and an arrow pointing towards the right is shown above time. the title on the y axis is left blank. what best explains the label that the student should use on the y-axis? amount, because as the amount of product decreases, the amount of reactant increases over time. reaction rate, because forward and backward reaction become equal at equilibrium. amount, because the amounts of reactants and products become constant after equilibrium is reached. reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.
Answers: 3
You know the right answer?
The reaction 2 NO(g) ⇌ N2(g) + O2(g) has a value of Keq = 2400 at a temperature of 2000 K. If 0.570...
Questions in other subjects:


Mathematics, 10.11.2021 02:20



Arts, 10.11.2021 02:20



Arts, 10.11.2021 02:20


![[N_2]_{eq}=0.094M](/tpl/images/0692/1303/f5b19.png)
![[O_2]_{eq}=0.094M](/tpl/images/0692/1303/b20e2.png)
![[NO]_{eq}=0.002M](/tpl/images/0692/1303/096e8.png)
![Keq=\frac{[N_2][O_2]}{[NO]^2}](/tpl/images/0692/1303/93add.png)
 due to the reaction extent (ICE procedure) we can write:
due to the reaction extent (ICE procedure) we can write:![Keq=\frac{x*x}{([NO]_0-2x)^2}](/tpl/images/0692/1303/d6f53.png)
![[NO]_0=\frac{0.570mol}{3.0L} =0.19M](/tpl/images/0692/1303/2b637.png)
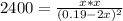
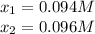
![[N_2]_{eq}=x=0.094M](/tpl/images/0692/1303/b3325.png)
![[O_2]_{eq}=x=0.094M](/tpl/images/0692/1303/3c9f2.png)
![[NO]_{eq}=0.19M-2x=0.19M-2(0.094M)=0.002M](/tpl/images/0692/1303/c2eca.png)



