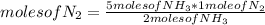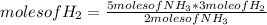N2(g) + 3H2(g) →2NH3(g)
At the end of the chemical reaction, 5 moles of NH3 are produced.
How...

Chemistry, 21.06.2020 22:57 Goldenstate32
N2(g) + 3H2(g) →2NH3(g)
At the end of the chemical reaction, 5 moles of NH3 are produced.
How many moles of N2 and H2 entered the reaction?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, happy121906
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2



Chemistry, 23.06.2019 07:30, danielahumajova6
How do you interpret a chromagram for what mixtures contain?
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 07.07.2019 04:00


Mathematics, 07.07.2019 04:00

Mathematics, 07.07.2019 04:00


Business, 07.07.2019 04:00


Chemistry, 07.07.2019 04:00

Spanish, 07.07.2019 04:00