(c)
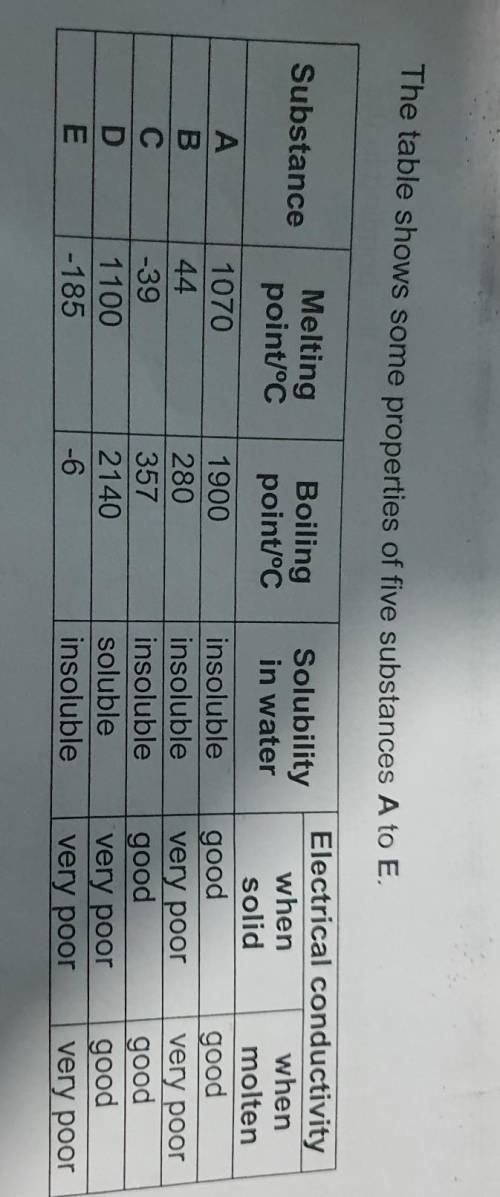
Suggest a reason why substance E does not conduct electricity in both solid and
molten sta...

Chemistry, 21.06.2020 20:57 lildeb2778
(c)
Suggest a reason why substance E does not conduct electricity in both solid and
molten states.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:30, arodavoarodavo
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3



You know the right answer?
Questions in other subjects:

Physics, 05.01.2020 12:31




Mathematics, 05.01.2020 12:31


Social Studies, 05.01.2020 12:31

Chemistry, 05.01.2020 12:31





