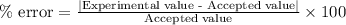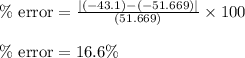Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 11:30, elijah1090
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
If the theoretical value for AH of the reaction HCl + NH 3 NH 4 Cl is -51.669 kJ/mol, but from your...
Questions in other subjects:


Business, 27.11.2019 22:31

Business, 27.11.2019 22:31




Advanced Placement (AP), 27.11.2019 22:31