
Chemistry, 20.06.2020 22:57 deojahnaeb37
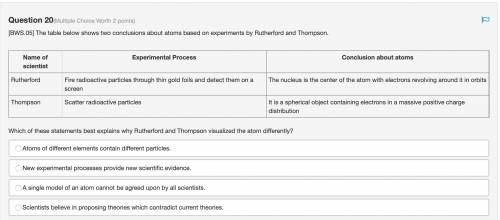
The table below shows two conclusions about atoms based on experiments by Rutherford and Thompson. Which of these statements best explains why Rutherford and Thompson visualized the atom differently? Atoms of different elements contain different particles. New experimental processes provide new scientific evidence. A single model of an atom cannot be agreed upon by all scientists. Scientists believe in proposing theories which contradict current theories.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1


Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
The table below shows two conclusions about atoms based on experiments by Rutherford and Thompson. W...
Questions in other subjects:

Mathematics, 20.12.2020 19:20




Health, 20.12.2020 19:20

Mathematics, 20.12.2020 19:20

English, 20.12.2020 19:20

History, 20.12.2020 19:20




