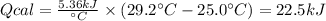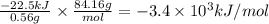Chemistry, 20.06.2020 12:57 stormserena
A 0.56 g sample of liquid C6H12 was combusted completely using excess oxygen inside a bomb (constant volume) calorimeter, with the products being carbon dioxide and liquid water. The calorimeter's heat capacity is 5.36 kJ °C-1. If the temperature inside the calorimeter increased from 25.0 °C to 29.2 °C, determine ΔrH for this reaction in kJ mol-1 (with respect to C6H12) at 298 K. Do not worry about how realistic the final answer is. You have 5 attempts at this question. TIP: To report an answer in scientific notation, enter it using the format "2.3E4", which means "2.3 x 104" (without the quotation marks)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
A 0.56 g sample of liquid C6H12 was combusted completely using excess oxygen inside a bomb (constant...
Questions in other subjects:




Social Studies, 28.09.2019 20:00




Mathematics, 28.09.2019 20:00

Physics, 28.09.2019 20:00

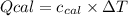
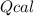 : heat absorbed by the calorimeter
: heat absorbed by the calorimeter : heat capacity of the calorimeter
: heat capacity of the calorimeter : change in the temperature
: change in the temperature